Smaller Paracetamol packs on the horizon as TGA clamps down with interim decision
March 21, 2023
What has changed
In late 2022, the Therapeutic Goods Administration (TGA) published an interim decision to reduce the maximum pack size for standard and modified release (MR) paracetamol products sold in supermarkets and convenience stores around Australia.
This decision follows on from increased concern growing following varied reports of the use of paracetamol products in an alarming increase in self-poisoning incidents in people >20 years of age. The TGA commissioned an independent expert report in 2022 which examined the incidence of serious injury and death from intentional paracetamol overdose. Paracetamol-containing products dominated the non-prescription analgesic market in pharmacies, selling around 40 million units a year in Australia in the most recent data. These products when sold in supermarkets and convenience stores lack the medical oversight necessary in purchase.
After examining multiple sources, the review revealed that intentional self-poisoning with paracetamol has been on the rise in Australia over the past decade, particularly among adolescents and young adults, with females being over represented. This trend is occurring in the context of a global increase in intentional self-poisoning cases among older children and adolescents (ages approximately 10 to 19) over the past decade. Paracetamol is the most commonly used medicine in the overdose of young Australians (around 50%) and this proportion has not increased despite the rise in self-poisoning activity.
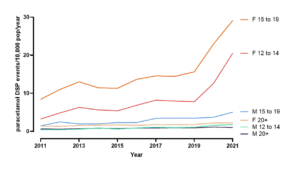
Data provided by NSW Poisons Information Centre, with estimated population adjusted annual rates for intentional self- poisoning events/10,000 pop generating calls to the Poisons Information Centre.
What are the dangers
The main factors that increase the risk of severe liver toxicity and death – such as delayed treatment, high doses, and ingestion of modified release (MR) paracetamol – have not changed and continue to occur in approximately 5 to 10% of cases of overdose. Despite the availability of effective treatments, there is still a risk of morbidity (acute liver injury/failure in 2 to 5% of cases) and fatalities (0.2 to 0.5%), which are primarily associated with these high- risk overdoses. Several interventions have been proposed to decrease the percentage of high-risk overdoses instead of preventing overdoses entirely. Although these outcomes are extremely unfortunate, they are relatively rare, with approximately 9 hospitalizations due to liver injury and 2 deaths per million population. Additionally, these occurrences are infrequent compared to the volume of paracetamol consumed in the community each year, with only 3 hospitalizations due to liver injury and less than 1 death per million units of paracetamol sold.
Evidence in the report found that over 50% of paracetamol taken in intentional self-poisoning was present in the home on the day of the incident. Only around 10% reported purchasing the drug on the day. This is quite alarming and raises valid concerns around the safe storage of medication in the home and adequate education around the uses of over-the-counter pharmaceuticals. At present, one-third of paracetamol sales occur in supermarkets and convenience stores, with multiple packets frequently purchased in the same transaction. However, given the impulsive and accessible nature of paracetamol poisoning, these transactional trends contribute only in part to the issue at hand.
What have other countries enforced regarding this trend
The review also concluded that paracetamol was found to likely be the first substance used for self-poisoning, particularly amongst young people and females. There are many factors to considering how this came to be, including accessibility, education and expense.
To address the issue, numerous countries such as the European Union, the United Kingdom, and New Zealand have implemented certain restrictions. For instance, they have placed more stringent controls on modified release (MR) formulations and limited the size and number of paracetamol packs available for purchase. According to the literature review, reducing the pack size has been shown to decrease fatalities caused by poisonings by approximately one-third. However, the impact on non-lethal outcomes may not be as significant.
What is the advice
There are four means of restriction and harm minimisation interventions to consider that are supported by overseas experience with regulation:
- Pack size restrictions. For example, maximum pack sizes for unscheduled products reduced from 20 to 12 or 16 tabs; S2 pack sizes reduced from 100 to 24 or 50.
- Pack number limits. Most (~95%) sales of paracetamol tablets involve the purchase of 1 or 2 packs. Making this the maximum number of packs that can be purchased in one transaction would almost certainly reduce home stockpiles, and likely also reduce the number of very large overdoses, which have much higher morbidity and risk of death.
- MR paracetamol restrictions. This product is designed for long-term use (e.g., for osteoarthritis), rather than for acute pain. Prescription only (S4) scheduling would be expected to reduce casual use of this more dangerous product and therefore overdoses.
- Age restrictions. An 18+ age restriction on the purchasing of over-the-counter analgesics would be expected to reduce poisonings among 10-17 year-olds.
The biggest impact is likely to come from MR paracetamol being made S4, followed by smaller pack size limits and pack number limits for S2, as both would be likely to reduce the number of grams of paracetamol routinely held in homes and thus the numbers of very large overdoses taken in impulsive self-poisonings.
There are also some non-medication specific recommendations to reduce self-poisoning.
- Use safe reporting guidelines for any communication around the harms associated with paracetamol (or any other) overdose. Any communication around the potential harms of paracetamol must comply with safe reporting guidelines and be rigorously evaluated prior to implementation.
- Maintain and expand support for aftercare services. All intentional self-poisonings should be offered appropriate care and Australian recommendations for aftercare (follow-up care and support after self-harm) implemented.
- Inform safer storage of medicines and reduced stockpiling of unwanted medicines. Generic messages around keeping medications and chemicals out of harm’s way might reduce intentional poisoning risks for children and adults.
In conclusion
The interim decision proposes to amend the Poisons Standard (which provides regulatory controls over medicine availability) to:
- Reduce the maximum size of packs available for General Sale (e.g. supermarkets and convenience stores) from 20 to 16 tablets or capsules
- Reduce the maximum size of packs available in pharmacies without supervision of a pharmacist (i.e. “Pharmacy Only” packs) from 100 to 32 tablets or capsules
- Make other pack sizes of up to 100 tablets or capsules only available under the supervision of a pharmacist (‘Pharmacist Only’ medicines).
It is acknowledged that additional information and data are needed in relation to understand the motivations and behaviours of young Australians in their deliberate attempts to self- harm. The TGA’s interim decision will be further considered and the guidelines are likely to be formally announced around April 2023.